黃廣慈 副研究員
慢性肝病與肝癌
脂質代謝與脂肪細胞分化
信息傳遞與基因調控
- 聯絡電話
(07) 731-7123 ext 8193
- 電子郵件信箱
huangkt@cgmh.org.tw
個人簡介
色素上皮衍生因子 (PEDF) 在非酒精性脂肪肝炎的功能性研究
非酒精性脂肪肝 (NAFLD) 是慢性肝病的主要成因,影響全世界約三分之一的人口,也常被視為代謝症候群在肝臟的具體表徵。肥胖與非酒精性脂肪肝的普遍程度和嚴重性有高度相關性。非酒精脂肪肝隨疾病進程區分為單純脂肪變性和非酒精性脂肪肝炎 (NASH),後者有演變成肝纖維化、甚至肝硬化的危險。雖然單純脂肪變性為三酸甘油酯的累積且大多不影響正常肝功能,具毒性的脂質代謝物會隨疾病進程逐漸增加並引發氧化壓力和慢性發炎,進一步造成胰島素抗性和過度的脂肪水解,最終導致疾病惡化。由於 NASH 目前無有效療法,研究致病的分子機制有助於發現預防和治療的新標的。
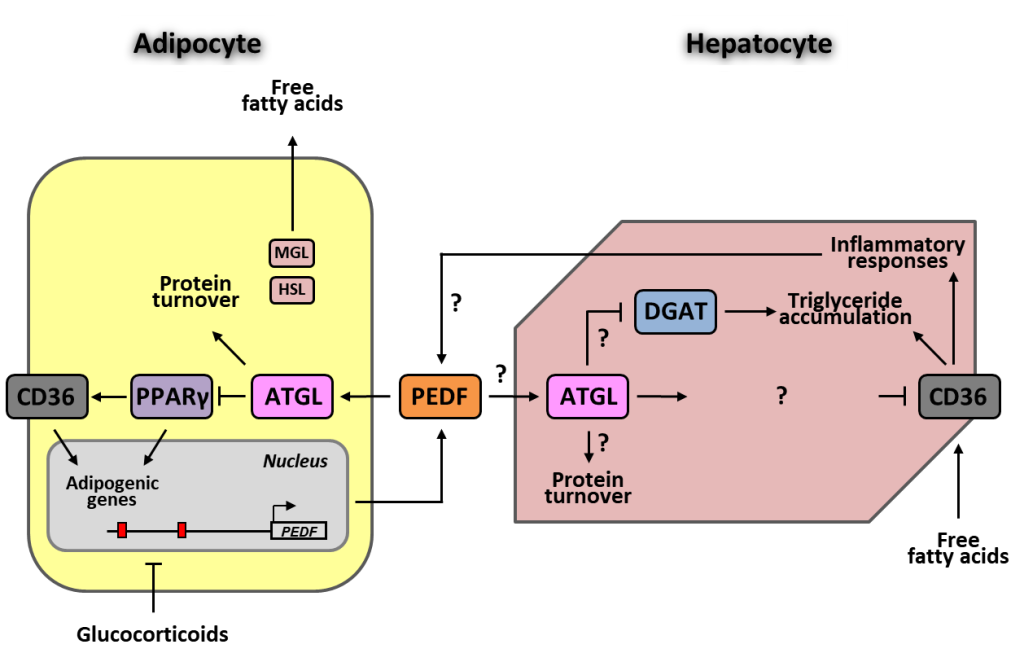
本研究室目前主要聚焦在色素上皮衍生因子 (PEDF) 這個多功能蛋白質,其中最為人熟知的是促進神經細胞分化和抑制血管新生。PEDF 在脂質代謝角色的建立是因發現它具有輔助脂肪三酸甘油酯水解酶 (ATGL) 的作用。我們近來的研究發現,PEDF 會透過 ATGL 調節脂肪酸轉位酶 (CD36) 的表現,進而抑制脂肪細胞分化。由於 CD36 是細胞攝取脂肪酸的重要酵素,我們假設 PEDF 也會透過 ATGL-CD36 的路徑引響肝臟脂質累積及其相關功能。我們也從 NASH 的小鼠模式中驗證 PEDF、ATGL、CD36 在疾病進程的調節角色,並進一步闡述 PEDF 訊息傳遞而影響下游與致病相關的機制,如油滴形成、脂肪細胞肥大和免疫反應等。我們也藉由抑制 CD36 活性,在小鼠 NASH 模型探討可能的治療途徑。
鈣網伴護蛋白 (calreticulin) 在肝纖維化過程的多重角色
肝纖維化在台灣和全世界都是嚴重影響健康的疾病,其主要特徵是細胞外基質過度累積造成的肝功能異常,肝硬化病人也是肝細胞癌的高危險群。肝纖維化是慢性肝損傷不斷修補的結果,損傷的肝細胞和滲入的發炎性免疫細胞會製造促進纖維化的因子,進而活化肝臟星狀細胞。星狀細胞活化會大量增生,並促進細胞外基質的生成。慢性肝損傷也會造成肝細胞死亡,趨使一些原本在細胞內的蛋白質暴露出來,這些蛋白質常表現不同於原本生理功能的作用,如本研究室所探討的,原本位於內質網中具有監控蛋白質摺疊並調節細胞鈣離子恆定功能的鈣網伴護蛋白 (calreticulin)。
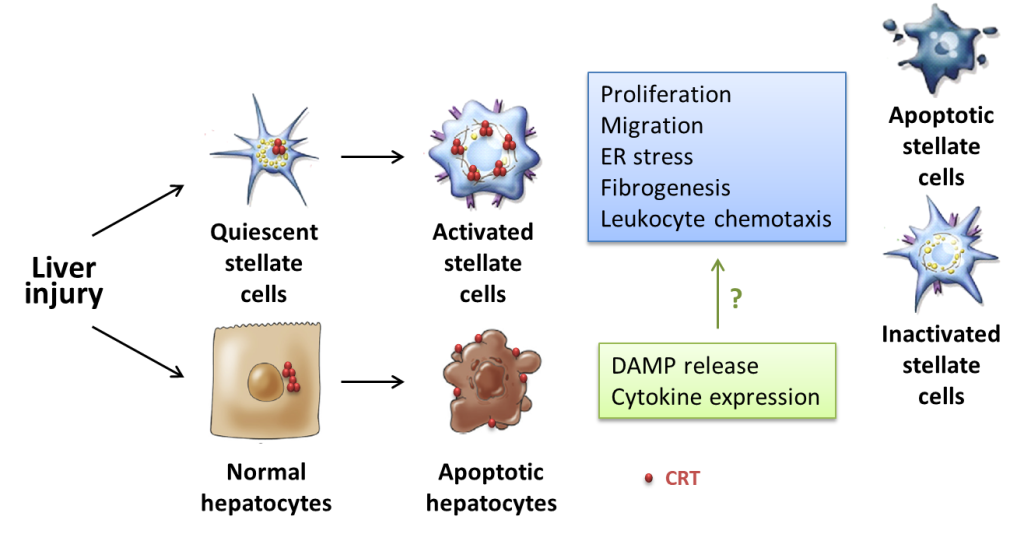 近來的文獻指出暴露於細胞外的鈣網伴護蛋白具有調節先天性免疫反應的作用。我們實驗數據顯示,肝纖維化小鼠的鈣網伴護蛋白表現量增加,觀察肝組織切片的免疫染色則發現,表現量增加集中於肝竇狀隙及其周圍受到損傷的肝組織,且有呈現於細胞表面的現象。有趣的是,在細胞實驗中,外加的鈣網伴護蛋白雖具有誘導促進發炎細胞激素生成的作用,但同時會減少星狀細胞活化標記的表現,並抑制星狀細胞增生。我們因此假設內質網和細胞外的鈣網伴護蛋白均參與肝纖維化的過程,但扮演著不同角色。因此我們進一步探討鈣網伴護蛋白在肝纖維化過程的功能,並評估細胞外鈣網伴護蛋白在肝纖維化小鼠模式中的生理效應。
近來的文獻指出暴露於細胞外的鈣網伴護蛋白具有調節先天性免疫反應的作用。我們實驗數據顯示,肝纖維化小鼠的鈣網伴護蛋白表現量增加,觀察肝組織切片的免疫染色則發現,表現量增加集中於肝竇狀隙及其周圍受到損傷的肝組織,且有呈現於細胞表面的現象。有趣的是,在細胞實驗中,外加的鈣網伴護蛋白雖具有誘導促進發炎細胞激素生成的作用,但同時會減少星狀細胞活化標記的表現,並抑制星狀細胞增生。我們因此假設內質網和細胞外的鈣網伴護蛋白均參與肝纖維化的過程,但扮演著不同角色。因此我們進一步探討鈣網伴護蛋白在肝纖維化過程的功能,並評估細胞外鈣網伴護蛋白在肝纖維化小鼠模式中的生理效應。
代表著作
Selected recent publications (Pubmed Search)
(† Contributed equally to the article; * Correspondence author of the article)
Chen CC, Nakano T, Hsu LW, Chu CY, Huang KT*. Early Lipid Metabolic Effects of the Anti-Psychotic Drug Olanzapine on Weight Gain and the Associated Gene Expression. Neuropsychiatric Disease and Treatment, 18:645-657 (2022)
Chen CC, Hsu LW, Chen KD, Chiu KW, Chen CL, Huang KT*. Emerging roles of calcium signaling in the development of non-alcoholic fatty liver disease. International Journal of Molecular Sciences, 23(1):256 (2022)
Huang KT*, Chen KD, Hsu LW, Kung CP, Li SR, Chen CC, Chiu KW, Goto S, Chen CL. Decreased PEDF promotes hepatic fatty acid uptake and lipid droplet formation in the pathogenesis of NAFLD. Nutrients, 12(1): 270 (2020)
Huang KT*, Hsu LW, Chen KD, Kung CP, Goto S, Chen CL. Decreased PEDF expression promotes adipogenic differentiation through the up-regulation of CD36. International Journal of Molecular Sciences, 19(12): E3992 (2018)
Huang KT*, Lin CC, Tsai MC, Chen KD, Chiu KW. Pigment epithelium-derived factor in lipid metabolic disorders. Biomedical Journal, 41(2): 102-108 (2018)
Huang KT, Kuo IY, Tsai MC, Wu CH, Hsu LW, Chen LY, Kung CP, Cheng YF, Goto S, Chou YW, Chen CL, Lin CC, Chen KD*. Factor VII-induced microRNA-135a inhibits autophagy and is associated with poor prognosis in hepatocellular carcinoma. Molecular Therapy Nucleic Acids, 9: 274-283 (2017)
Daubriac J†, Pandya UM†, Huang KT† (co-first authors), Pavlides SC, Gama P, Blank SV, Shukla P, Crawford SE, Gold LI*. Hormonal and growth regulation of epithelial and stromal cells from the normal and malignant endometrium by pigment epithelium-derived factor. Endocrinology, 158(9): 2754-2773 (2017)
Chen CC, Hsu LW, Huang KT, Goto S, Chen CL, Nakano T*. Overexpression of Insig-2 inhibits atypical antipsychotic-induced adipogenic differentiation and lipid biosynthesis in adipose-derived stem cells. Scientific Reports, 7(1): 10901 (2017)
Chen KD, Huang KT, Tsai MC, Wu CH, Kuo IY, Chen LY, Hu TH, Chen CL, Lin CC*. Coagulation factor VII and malignant progression of hepatocellular carcinoma. Cell Death & Disease, 7: e2110 (2016)
Chen KD*, Huang KT, Lin CC, Weng WT, Hsu LW, Goto S, Nakano T, Lai CY, Kung CP, Chiu KW, Wang CC, Cheng YF, Ma YY, Chen CL*. MicroRNA-27b Enhances the Hepatic Regenerative Properties of Adipose-Derived Mesenchymal Stem Cells. Molecular Therapy Nucleic Acids, 5: e285 (2016)
Huang KT, Tan D, Chen KH, Walker AM*. Blockade of estrogen-stimulated proliferation by a constitutively-active prolactin receptor having lower expression in invasive ductal carcinoma. Cancer letters, 358(2): 152-160 (2015)
研究團隊
孔昭蘋
學術履歷
EDUCATION:
2002 – 2008 Doctor of Philosophy
Biomedical Sciences, University of California, Riverside, Riverside, CA, USA
1996 – 1998 Master of Science
Biochemistry, National Yang-Ming University, Taipei, Taiwan
1992 – 1996 Bachelor of Science
Chemical Engineering, National Tsing Hua University, Hsinchu, Taiwan
PROFESSIONAL EXPERIENCE:
2020 – present Associate Research Fellow
2013 – 2020 Assistant Research Fellow
Institute for Translational Research in Biomedicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
My current interest is to identify potential molecular targets (proteins, small RNAs) involved in the initiation and progression of lipid metabolic disorders, chronic liver diseases, and hepatocellular carcinoma.
2009 – 2013 Postdoctoral Fellow
Department of Medicine, New York University Medical Center, New York, NY, USA
The research project was focused on hormonal regulation of the cell cycle inhibitor p27/kip1 in ubiquitin-proteasome degradation system in endometrial carcinoma. I also participated in designing and testing small molecule inhibitors of an E3 ligase (Skp2) that targets p27 for degradation. The other project included a growth-inhibitory protein, pigment epithelium-derived factor, whose expression and mechanisms of action in endometrial cancer were studied.
2003 – 2008 Graduate Research Assistant
Division of Biomedical Sciences, University of California, Riverside, Riverside, CA, USA
The dissertation project was to investigate the role of a group of naturally occurring, constitutively active prolactin receptors in human prostate and breast cancers. I also actively participated in three additional projects: 1) characterizing a secreted prolactin receptor isoform which counteracts prolactin action, 2) localizing prolactin receptor isoforms in mouse mammary ducts, and 3) searching for potential prolactin binding factors in human milk.
ACADEMIC APPOINTMENT:
2014 – 2015 Adjunct Assistant Professor
Bachelor Program of Medical Engineering, Cheng Shiu University, Kaohsiung, Taiwan
Lecturer in 1) Biochemistry, 2) Introduction to Biotechnology, 3) Bio-electro-mechanical Industry Analysis
2010 – 2013 Teaching Assistant
Sackler Institute of Graduate Biomedical Sciences, New York University, New York, NY, USA
Leader in discussion sessions: Foundations of Cell and Molecular Biology II (TGF-β Signaling)
2006 – 2007 Teaching Assistant
Department of Biology, University of California, Riverside, Riverside, CA, USA
Laboratory sessions; discussion sessions; office hours; grading of laboratory notebooks and exams: Biology 5B (Introduction to Organismal Biology)
PROFESSIONAL ACTIVITIES:
2017 – present Committee member, Basic Science Committee, International Liver Transplantation Society
2006 – 2013 Associate member, Endocrine Society
AWARDS AND HONORS:
2018 Session moderator/Oral speaker, The 2018 Joint International Congress of ILTS, ELITA & LICAGE, Lisbon, Portugal.
2016 Oral speaker, International Liver Transplantation Society 22nd Annual International Congress, Seoul, Korea
2015 Oral speaker, International Liver Transplantation Society 21st Annual International Congress, Chicago, IL, USA
2006 – 2009 Breast Cancer Research Program Predoctoral Traineeship Award
Awarded by the Department of Defense (USA) for three-year predoctoral research titled “The Role of Constitutively Active Prolactin Receptors in the Natural History of Breast Cancer”.
2007 Award for Outstanding Student Research Presentation, Division of Biomedical Sciences, University of California, Riverside
2005 Joy K Tilton Cancer Research Award, Division of Biomedical Sciences, University of California, Riverside
2004 Cancer Federation Award, Division of Biomedical Sciences, University of California, Riverside. (Featured in Press-Enterprise)
2002 – 2003 Dean’s Fellowship, Division of Biomedical Sciences, University of California, Riverside
RESEARCH INTERESTS:
1. Molecular pathogenesis of chronic liver diseases, including cirrhosis, steatohepatitis and hepatocellular carcinoma
2. Molecular mechanisms of adipogenic/osteogenic differentiation
3. Functional characterization of immunogenic danger signals in the liver
