楊政霖 研究員
細胞和分子神經科學
神經細胞 DNA 修復
星形膠質細胞病理生理學
缺血性中風之治療與機制
- 聯絡電話
(07) 731-7123 ext 8593
- 電子郵件信箱
jyang@cgmh.org.tw
個人簡介
探討涉及調控神經細胞和星形膠質細胞中DNA修復蛋白的信號機轉是實驗室過去數年的研究主軸。我們的目標是為了解衰老、神經失調和缺血性中風等過程中產生DNA損傷的致病因子的機制,然後通過提高DNA修復效率來應對DNA損傷以增加神經細胞存活。開發神經系統疾病的治療方法是我們實驗室的最終目標。
目前,我的實驗室亦協助或與醫生合作發展新的實驗和計畫,試圖將神經退化性疾病、大腦缺血性中風、血管和腎性認知病變領域的基礎研究工作與的臨床治療聯繫起來。 此外,涉及神經發炎的星形膠質細胞和線粒體功能障礙也是目前的研究目標。
經由提高APE1表現而提高神經細胞氧化DNA修復效率
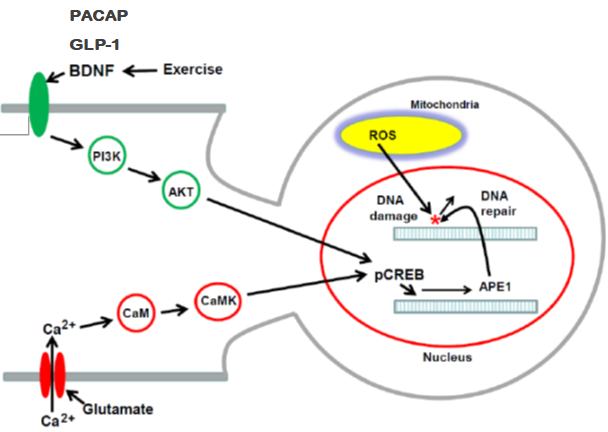 不再有絲分裂的神經細胞是高度分化的細胞,其表現出經常性興奮性電位訊號傳遞、高代謝率和大量的轉錄活性。這些細胞中的高代謝活性產生相對大量的活性氧物質 (ROS),其可破壞脂質、蛋白質和DNA。由於神經細胞代謝率較高,其受到氧化反應的刺激而產生氧化性DNA損傷。細胞核DNA和線粒體DNA中,氧化性DNA損傷的累積是神經退化性疾病和正常衰老的病原體。因此,DNA修復能力對神經細胞的正常功能和存活至關重要。 鹼基切除修復(BER)途徑通常被認為是修復DNA的氧化損傷、脫氨、烷基化和單鏈斷裂的主要機制,其涉及多種酵素和路徑過程,例如DNA糖基化酶、脫嘌呤/脫嘧啶核酸內切酶1(APE1)、DNA聚合酶β和連接酶。我們之前的研究顯示,APE1能夠經由氧化反應、神經遞質(谷氨酸)、腦源性神經營養因子(BDNF)和類昇糖素胜肽-1 (GLP-1) 而增強,以提高DNA修復效率,並非BER的其他修復酶。我們的研究發現氧化反應、谷氨酸、BDNF和GLP-1的所有下游信號傳導都朝向CaMK-CREB或Akt-CREB訊號鏈以增強APE1之表現。我們提出CREB信號傳遞鏈為提高APE1表現和氧化DNA修復效率的重要途徑,可作為神經退行性疾病、缺血性中風、癲癇發作和許多其他神經系統疾病的治療方向。
不再有絲分裂的神經細胞是高度分化的細胞,其表現出經常性興奮性電位訊號傳遞、高代謝率和大量的轉錄活性。這些細胞中的高代謝活性產生相對大量的活性氧物質 (ROS),其可破壞脂質、蛋白質和DNA。由於神經細胞代謝率較高,其受到氧化反應的刺激而產生氧化性DNA損傷。細胞核DNA和線粒體DNA中,氧化性DNA損傷的累積是神經退化性疾病和正常衰老的病原體。因此,DNA修復能力對神經細胞的正常功能和存活至關重要。 鹼基切除修復(BER)途徑通常被認為是修復DNA的氧化損傷、脫氨、烷基化和單鏈斷裂的主要機制,其涉及多種酵素和路徑過程,例如DNA糖基化酶、脫嘌呤/脫嘧啶核酸內切酶1(APE1)、DNA聚合酶β和連接酶。我們之前的研究顯示,APE1能夠經由氧化反應、神經遞質(谷氨酸)、腦源性神經營養因子(BDNF)和類昇糖素胜肽-1 (GLP-1) 而增強,以提高DNA修復效率,並非BER的其他修復酶。我們的研究發現氧化反應、谷氨酸、BDNF和GLP-1的所有下游信號傳導都朝向CaMK-CREB或Akt-CREB訊號鏈以增強APE1之表現。我們提出CREB信號傳遞鏈為提高APE1表現和氧化DNA修復效率的重要途徑,可作為神經退行性疾病、缺血性中風、癲癇發作和許多其他神經系統疾病的治療方向。
星形膠質細胞在缺血性中風治療中的角色
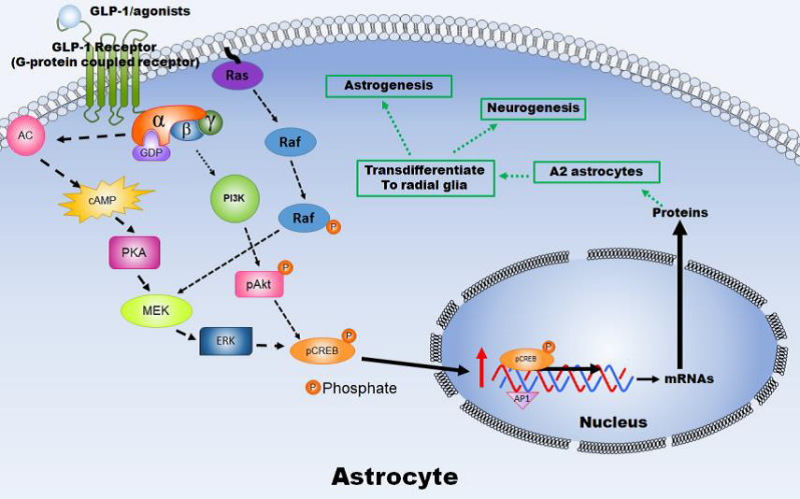 星形膠質細胞是哺乳動物中樞神經系統(CNS)中最豐富的細胞,並且在維持動態平衡和大腦正常功能方面發揮多重作用。星形膠質細胞的細胞和分子內容物、形態和功能會受各種CNS損傷而改變,而被稱為“反應性星形膠質細胞”。反應性星形膠質細胞的作用尚未明確定義,其對CNS損傷後的恢復有好有壞。星形膠質細胞的所有生理和細胞特性包括耐缺氧、分泌神經營養因子、抗興奮性毒性、神經膠質瘢痕形成、介導神經細胞生成和突觸生成、增強血管生成、維持BBB功能等,都顯示出星形膠質細胞是缺血性中風、甚至其他神經系統疾病的潛在治療標的。我們目前的研究已經觀察到缺血性中風周邊區 (penumbral area) 的星形膠質細胞能夠轉分化成放射狀膠質細胞,我們正在研究涉及星形膠質細胞存活和轉分化的可能機制。提高星形膠質細胞活力並有效調節反應性星形膠質細胞對缺血性中風後的有益影響可能是開發治療方法的新方向。
星形膠質細胞是哺乳動物中樞神經系統(CNS)中最豐富的細胞,並且在維持動態平衡和大腦正常功能方面發揮多重作用。星形膠質細胞的細胞和分子內容物、形態和功能會受各種CNS損傷而改變,而被稱為“反應性星形膠質細胞”。反應性星形膠質細胞的作用尚未明確定義,其對CNS損傷後的恢復有好有壞。星形膠質細胞的所有生理和細胞特性包括耐缺氧、分泌神經營養因子、抗興奮性毒性、神經膠質瘢痕形成、介導神經細胞生成和突觸生成、增強血管生成、維持BBB功能等,都顯示出星形膠質細胞是缺血性中風、甚至其他神經系統疾病的潛在治療標的。我們目前的研究已經觀察到缺血性中風周邊區 (penumbral area) 的星形膠質細胞能夠轉分化成放射狀膠質細胞,我們正在研究涉及星形膠質細胞存活和轉分化的可能機制。提高星形膠質細胞活力並有效調節反應性星形膠質細胞對缺血性中風後的有益影響可能是開發治療方法的新方向。
代表著作
Selected Recent Publications (Pubmed Search)
Yang JL, Chen WY, Mukda S, Yang YR, Sun SF, Chen SD*. Oxidative DNA Damage is Concurrently Repaired by Base Excision Repair (BER) and Apyrimidinic Endonuclease 1 (APE1)-initiated Nonhomologous End Joining (NHEJ) in Cortical Neurons. Neuropathology and Applied Neurobiology, 46(4): 375-390 (2020).
Yang JL, Yang YR, Chen SD*. The potential of drug repurposing combined with reperfusion therapy in cerebral ischemic stroke: A supplementary strategy to endovascular thrombectomy. Life Sciences, 236: 116889 (2019).
Yang JL, Mukda S, Chen SD*. Diverse roles of mitochondria in ischemic stroke. Redox Biology, 16: 263-275 (2018).
Yang JL, Chen WY, Chen SD*. The Emerging Role of GLP-1 Receptors in DNA Repair: Implications in Neurological Disorders. International Journal of Molecular Sciences, 18(9): 1861 (2017).
Chen WY. Li LC, Yang JL*. Emerging Roles of IL-33/ST2 Axis in Renal Diseases. International Journal of Molecular Sciences, 18(4): 783 (2017).
Yang JL, Chen WY, Chen YP, Kuo CY, Chen SD*. Activation of GLP-1 receptor enhances neuronal base excision repair via PI3K-AKT-induced expression of apurinic/apyrimidinic endonuclease 1. Theranostics, 6(12): 2015-2027 (2016).
Chuang YC, Lin TK, Yang DI, Yang JL, Liou CW, Chen SD*. Peroxisome proliferator-activated receptor-gamma dependent pathway reduces the phosphorylation of dynamin-related protein 1 and ameliorates hippocampal injury induced by global ischemia in rats. Journal of Biomedical Sciences, 12;23(1):44 (2016).
Chen WY*, Chang YJ, Su CH, Tsai TH, Chen SD, Hsing CH, Yang JL*. Upregulation of Interleukin-33 in obstructive renal injury. Biochemical and Biophysical Research Communications, 473(4): 1026-1032 (2016).
Hsieh MC, Tsai CY, Liao MC, Yang JL, Su CH, Chen JH*. Quantitative Susceptibility Mapping-Based Microscopy of Magnetic Resonance Venography (QSM-mMRV) for In Vivo Morphologically and Functionally Assessing Cerebromicrovasculature in Rat Stroke Model. PLoS One, 14;11(3):e0149602 (2016).
Chuang YC†, Yang JL†, Yang DI, Liou CW, Chen SD*. Roles of Sestrin2 and Ribosomal Protein S6 in Transient Global Ischemia-Induced Hippocampal Neuronal Injury. International Journal of Molecular Sciences, 16:26406-26416 (2015).
Tsai TH, Sun CK, Su CH, Sung PH, Chua S, Zhen YZ, Leu S, Chang HW, Yang JL, Yip HK*. Sitagliptin attenuated brain damage and cognitive impairment in mice with chronic cerebral hypo-perfusion through suppressing oxidative stress and inflammatory reaction. Journal of Hypertension , 33(5):1001-13 (2015).
研究團隊
劉佩禎
李姝瑩
盧宣聿
學術履歷
EDUCATION:
1999 – 2006 Ph.D.
Department of Biology, Neuroscience Program,
University of Texas at San Antonio, San Antonio, Texas USA
PROFESSIONAL EXPERIENCE:
2021/8 ~ present Professor
2018/8 ~ 2021/7 Associate Professor
2011/8 ~ 2018/7 Assistant Professor
Institute for Translational Research in Biomedicine,
Kaohsiung Chang Gung Memorial Hospital,
Kaohsiung, Taiwan
2007/1 ~ 2011/7 Postdoctoral Research Fellow
Laboratory of Molecular Gerontology and Laboratory of Neurosciences,
National Institute on Aging, National Institute of Health, Baltimore, Maryland USA
2006/1 ~ 2006/11 Research Scientist Associate
Department of Biology
University of Texas at San Antonio, San Antonio, Texas USA
2002/9 ~ 2005/12 Instructor of Cellular Biology Laboratory
Department of Biology
University of Texas at San Antonio, San Antonio, Texas USA
2001/9 ~ 2001/12 Instructor of Molecular Biology Laboratory
Department of Biology
University of Texas at San Antonio, San Antonio, Texas USA
2000/9 ~ 2002/5 Instructor of Laboratory Investigations in Biology
Department of Biology
University of Texas at San Antonio, San Antonio, Texas USA
1997/1 ~ 1999/8 Graduate Research Assistant
Department of Biology
University of Texas at San Antonio, San Antonio, Texas USA
AWARDS AND RESEARCH SUPPORT:
2009 Fellow Achievement Research Excellence Award, National Institute of Health
2001 Graduate Travel Award
1998 University Graduate Small Grant
1997 University Graduate Small Grant
RESEARCH INTERESTS:
1. DNA Damage and Repair in Neurons and Astrocytes
2. Roles of GLP-1 in Neurons and Glial Cells
3. Diagnosis and Therapy on Alzheimer’s Disease and Ischemic Stroke
